EASY 1 HOUR MYCOPLASMA DETECTION IN CELL THERAPY SAMPLES
INTRODUCTION
While pharmacopoeias specify a test for mycoplasma contamination must be conducted prior to releasing product manufactured in the presence of eukaryotic cells (1), current compendial methods require ≥ 28 days to generate test results. Waiting 28 days or longer to release product isn’t a feasible option for most autologous cell and gene therapy processes where patients are waiting to be infused and time is critical to effective treatment.
Conventional nucleic acid testing (NAT) methods offer a faster alternative but they often require specialized laboratories, elevated skill levels and significant training to interpret results. Because of these complexities, many cell and gene therapy companies outsource their mycoplasma testing to a third party laboratory. While often this solution provides results faster than compendial methods, the cost of outsourced labor can be high.
The BIOFIRE® Mycoplasma solution utilizes the FILMARRAY® 2.0 Industry instrument and a closed pouch PCR test to detect the presence of >130 species of mycoplasma. The system provides sample to answer in ~1 hour with little technical training required. This provides options for bringing mycoplasma testing in-house, thus saving time and outsourcing costs.
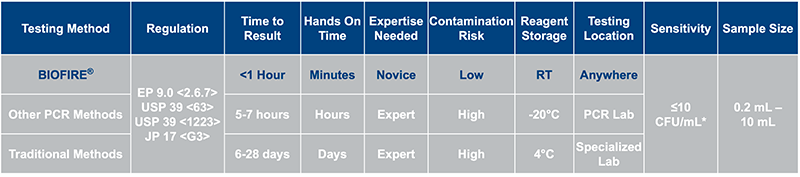
*10 mL protocol
The data summarized in this White Paper is from two cell therapy manufacturers that evaluated the BIOFIRE® FILMARRAY® 2.0 Industry System and presented their results at the 2022 Association for the Advancement of Blood and Biotherapies annual conference (2). Samples were evaluated with up to 3 compendial mycoplasma strains in the presence of high-density chimeric antigen receptor T (CAR T) cells or autologous cultured chondrocytes. Studies were designed to assess product interference (false-positive rates) and detection (false-negative rates). In addition, data from numerous internal studies that tested product compatibility & low level inoculation on commonly used media and raw materials is presented.
THE VALUE OF RAPID, EASY MYCOPLASMA TESTING
Because the BIOFIRE® system is so easy to use, manufacturers who previously lacked the resources to perform mycoplasma testing in-house can now easily perform mycoplasma screening at-line with little specialized equipment or training saving on costly outsourced laboratory testing.
Cell and gene therapy manufacturers today waiting hours or weeks for results using complex testing methods can realize significant value with a more rapid, simplified approach to mycoplasma testing using BIOFIRE® FILMARRAY 2.0 Industry.

The BIOFIRE® mycoplasma test can be performed by anyone, anywhere at anytime allowing manufacturers to take critical quality testing out of the lab and closer to manufacturing to realize the following benefits:• 1-hour time to result for faster patient treatment
• Simplified training requirements
• Lower expertise required
• Objective results
• Less human error risk/improved Data Integrity
• Does not require molecular lab
 .
.
BIOFIRE FILMARRAY 2.0 INDUSTRY SYSTEM
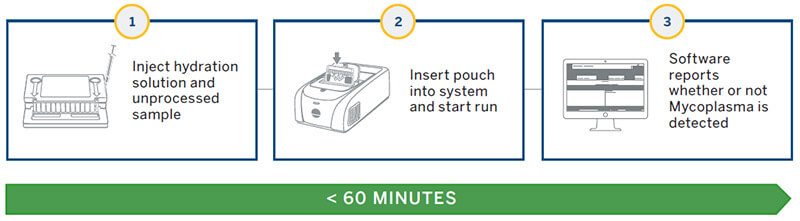
The BIOFIRE system utilizes the FILMARRAY 2.0 Industry instrument and next generation PCR testing in a closed pouch to detect the presence of mycoplasma (Figures 1 and 2). The disposable BIOFIRE Mycoplasma pouch contains all the necessary reagents for automated PCR and analyte detection in order to isolate, amplify and detect over 130 different mycoplasma species (Figure 2).
Several controls are integrated into the pouch to ensure the quality of the results including a total process control, reverse transcription control, and PCR I and II controls. The instrument & software process the pouch with results in less than an hour.
The FILMARRAY 2.0 Industry software (21 CFR Part 11 compliance ready) performs all the complex meta-analysis and provides presence/absence results as either “Mycoplasma Detected” or
“Mycoplasma Not Detected”.
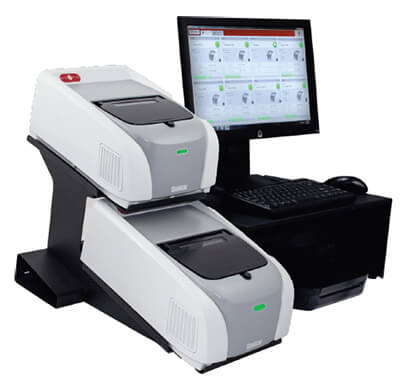 Figure 1:
Figure 1:
FILMARRAY® 2.0 Industry instrument performs the extraction, amplification and detection (25.4 x 39.3 x 16.5 cm WxDxH). The system comes standard with 2 instruments; up to 8 instruments can be connected to a single PC.
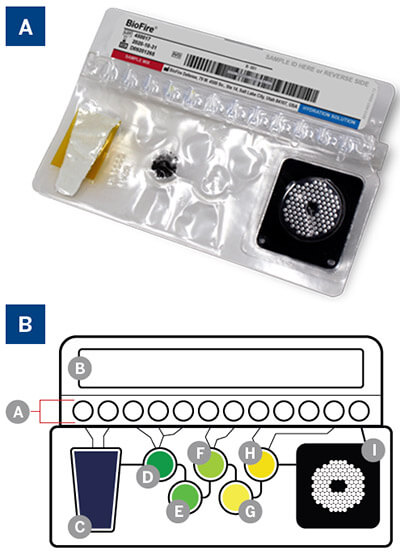
Figure 2:
A. BIOFIRE® Mycoplasma pouch.
B. Pouch diagram: (A) Fitment with freezedried reagents; (B) Plungers-deliver reagents to blisters; (C) Sample lysis and bead collection; (D) Wash; (E) Magnetic bead collection blister; (F) Elution; (G) Multiplex Outer PCR blister; (H) Dilution blister; (I) Inner Nested PCR array
SAMPLE PROTOCOLS
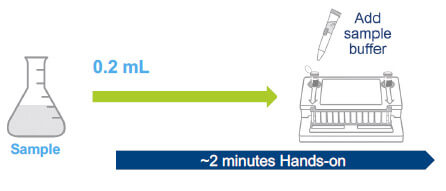
Two standard protocols have been designed to detect mycoplasma contamination (3). These include a 0.2 mL direct assay that can be used for in-process control testing with a validated LOD of ~≤ 30 CFU/ mL, and a 10 mL release test protocol that concentrates the sample using centrifugation and has a validated LOD of ≤ 10 CFU/mL.
 .
.
SUITABLE FOR IN-PROCESS CONTROLS LOD ≤ 30 CFU/mL
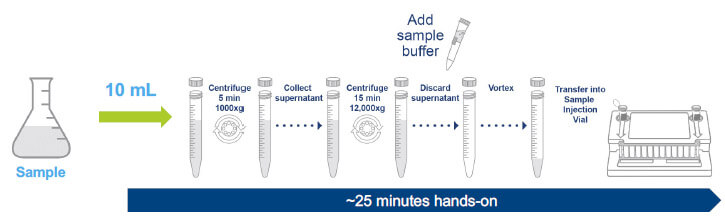 SUITABLE FOR RELEASE TESTING LOD ≤ 10 CFU/mL
SUITABLE FOR RELEASE TESTING LOD ≤ 10 CFU/mL
These protocols were customized by both evaluators during the study (figure 4). Evaluator A deviated from the manufacturer recommendations with customized sample volumes and evaluator B tested the 10 mL protocol bypassing the initial low-speed centrifugation due to the low concentration of cells in their product matrix. Following sample pre-processing, samples were then loaded onto a fully prepared and hydrated pouch and run on the FILMARRAY 2.0 Industry instrument.